Marko Hapke - Research Results.
59.
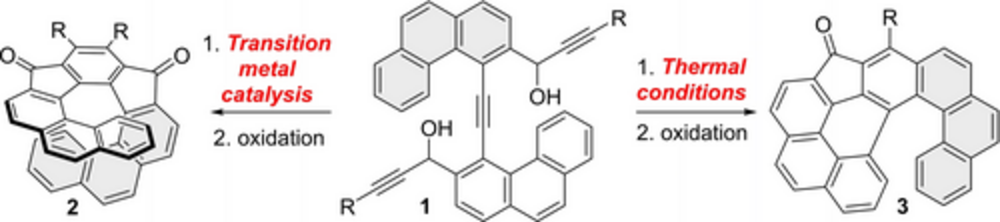
“Cyclotrimerization Approach to Symmetric [9]-Helical Indenofluorenes: Diverting Cyclization Pathways”
T. Cadart, T. Gläsel, I. Císařová, R. Gyepes, M. Hapke, M. Kotora
Chem. Eur. J. 2023, 29, e202301491
Link: https://doi.org/10.1002/chem.202301491, opens an external URL in a new window
58.
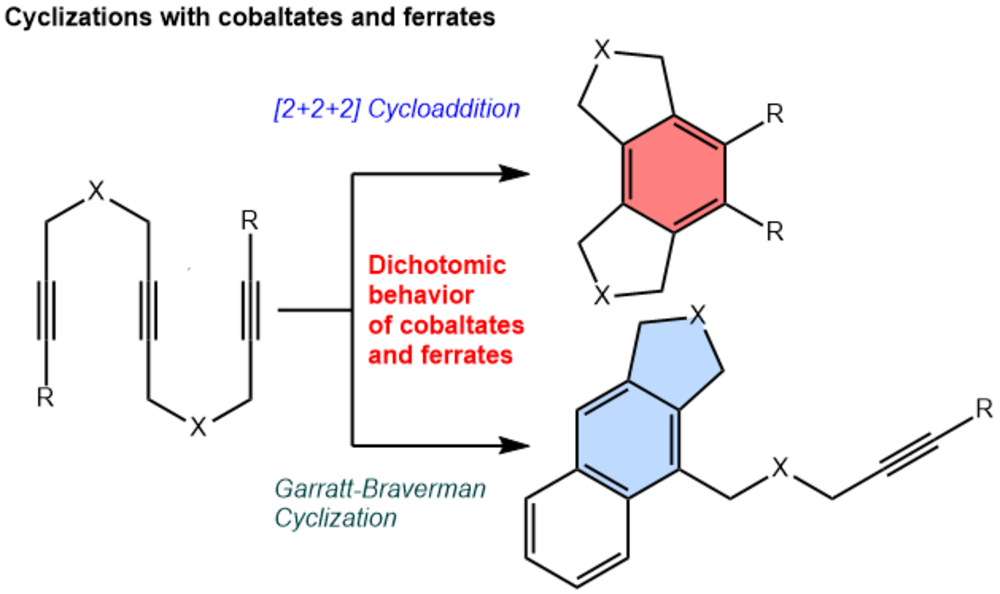
“Cobalt and Iron Metallates as Catalysts for Cyclization Reactions of Triynes: [2+2+2] Cycloaddition vs. Garratt-Braverman Reaction”
B. Baumann, H. Lange, F. Seeberger, P. Büschelberger, R. Wolf, M. Hapke
Mol. Catal, opens an external URL in a new window. 2023, 550, 113482
Link: https://doi.org/10.1016/j.mcat.2023.113482, opens an external URL in a new window
57.
“Atroposelective Ir-catalyzed C-H borylation of phthalazine heterobiaryls”
P. Stehrer, A. Spannenberg, M. Hapke
J. Org. Chem. 2023, 88, 14222-14226
Link: https://doi.org/10.1021/acs.joc.3c01534, opens an external URL in a new window
56.
„Recent Advances in Catalytic Chemical Recycling of Polyolefins”
K. Faust, opens an external URL in a new window, P. Denifl, opens an external URL in a new window, M. Hapke, opens an external URL in a new window
ChemCatChem 2023, 15, e202300310
Link: https://doi.org/10.1002/cctc.202300310, opens an external URL in a new window
55.
“Cobalt Catalysts for [2+2+2] Cycloaddition Reactions: Isolated Precatalysts and in situ Generated Catalysts” (invited review)
T. Gläsel, B. Baumann, M. Hapke
Chem. Rec. 2021, 21, 3727-3745
Special Issue: Recent Advances in Transition‐Metal Catalysis, opens an external URL in a new window
Link: https://doi.org/10.1002/tcr.202100273, opens an external URL in a new window
54.
“CpCo(III) precatalysts for [2+2+2] cycloadditions”
F. Fischer, M. Eder, M. Hapke
Catalysts 2021, 11, 596
Link: https://doi.org/10.3390/catal11050596, opens an external URL in a new window
53.
“Synthesis of Phosphinines from CoII-Catalyzed [2+1 2+2] Cycloaddition Reactions”
T. Gläsel, H. Jiao, M. Hapke
ACS Catal. 2021, 11, 13434-13444
Link: https://doi.org/10.1021/acscatal.1c03483, opens an external URL in a new window
 Go to JKU Homepage
Go to JKU Homepage


