Spatially resolved impedance spectroscopy on a microchannel for monitoring chemical reactions
Supervisor: Univ.-Prof. Dr. Marco Da Silva
Co-Supervisor: Dipl.-Ing. Dr. Andreas Tröls
![[Translate to Englisch:] Alexander Galler](/fileadmin/_processed_/9/f/csm_Galler_Foto_6f7592d035.png)
Measuring on a microchannel has many advantages, in addition to the low sample consumption, which is particularly relevant in medicine, it is also possible to measure chemical reactions with high precision. The aim of any circuit is to realise a so called lab-on-a-chip, that combines several work steps.
The work therefore deals with the production of a prototype and the subsequent spatially resolved application of electrical impedance spectroscopy to measure a chemical reaction on a microchannel. The chemical reaction is a neutralisation reaction of hydrochloric acid (HCl) and caustic soda (NaOH), which produces salt water. The prototype consists of two inlets and one outlet, which are connected in the form of a Y-channel through which the fluids to be measured are pumped. Four gold electrodes are attached to the microchannel itself, which are read out separately via an impedance analyser.
Various measurements were carried out with the manufactured prototype to analyse the influence of the measurement parameters such as flow velocity and conductivity of the fluid to be measured as well as the frequency range of the measuring device on the impedance spectrum. It was found that the flow velocity has hardly any influence on the result of the measurement, but the influence of the conductivity is significant on the magnitude and phase response.
To analyse the chemical reaction, only the impedance of individual frequency points was considered instead of the entire frequency spectrum. The relative deviations of the admittance of the electrodes were used as the basis for calculation in order to draw conclusions about the progress of the reaction. A comparison of the admittances shows that the later the reaction was measured, the greater the relative deviation, which corresponds to the progress of the reaction.
It was therefore shown that chemical reactions can be analysed using the prototype developed.
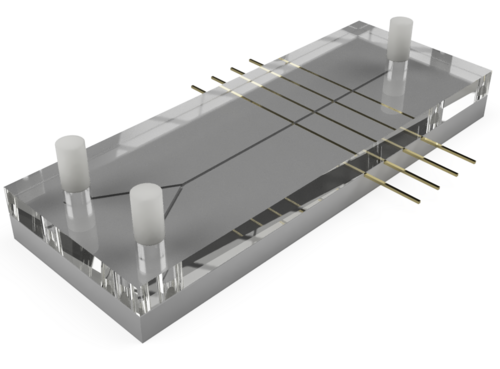 Fig. 1: Schematic illustration of the prototype
Fig. 1: Schematic illustration of the prototype
Keywords: Impedance spectroscopy, Microchannel, Production of a prototype, Lab-on-a-chip
August 26, 2024
 Go to JKU Homepage
Go to JKU Homepage


